Industry Solutions
We provide flexible, end-to-end solutions that cover the full product lifecycle. Our services are designed to reduce complexity, manage risk and accelerate access to markets worldwide.


MMAP Platform
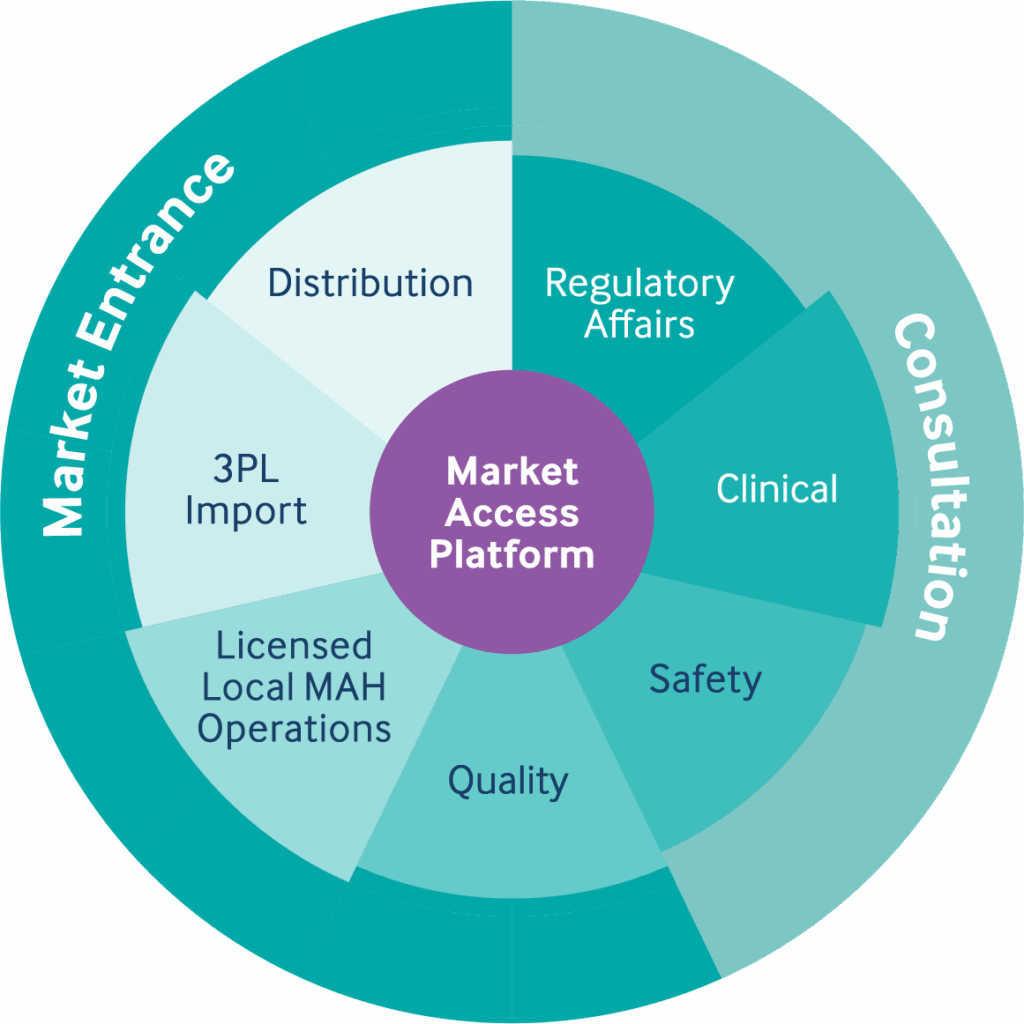
GRP’s MAH Market Access Platform (MMAP) provides integrated End-to-End Core MAH services (regulatory, safety, quality, importation, storage, local manufacturing) and Market Access services (distribution and commercialization) to accelerate market access of companies and their innovative products to Japan.
The MMAP also enables Japanese companies to expand globally without setting up costly infrastructure. GRP has licensed MAH representation in Japan, US, Brazil, China, Mexico and South Korea.
Our services include:
- Integrated solution combining regulatory, clinical, safety, quality, logistics and distribution, sales and service
- 3PL and supply chain management for import, storage and delivery
- Proven end-to-end access model validated in Japan and scaled globally for SMEs
MAH Solutions
Regulatory Consulting & Submission

- Strategic regulatory planning tailored to Japan and other key markets
- PMDA consultation meeting preparation and follow-up
- Guidance on dossier requirements, electronic submissions and regulatory strategy alignment
- Dossier preparation and electronic CTD submissions
Compliance & Risk Management

- Quality management systems aligned with PMDA and ICH standards
- Pharmacovigilance and safety monitoring, including Risk Management Plans (RMPs)
- Post-marketing safety updates and compliance oversight
Clinical Operations

- Protocol development and site selection
- Clinical monitoring, data collection and trial management
- Local regulatory submissions and GCP compliance support
Market Access & Commercialization

- Support with pricing, reimbursement and health authority interactions
- Multi-country registration management for pharmaceuticals, devices, diagnostics, cosmetics and quasi-drugs
- Distribution and end-to-end access to end users
MAH Capabilities
As a licensed Marketing Authorization Holder (MAH) in Japan, we provide comprehensive solutions that cover the full product lifecycle.
- Licensed MAH representation in Japan, US, Brazil, China, Mexico and South Korea
- Local representation for regulatory, safety and quality compliance
- Lifecycle management and post-market support
MAH Solutions
Our services include:
- Regulatory strategy and product registration
- Pharmacovigilance and safety reporting
- Quality management and compliance oversight
- Logistics and distribution support
- Sales and service
- Post-market monitoring and lifecycle management

Get in Touch
Ready to bring your products to Japan? Our regulatory experts are here to guide you every step of the way.